Karl Fischer (KF) titration is one of the most specific water determination techniques available and is widely used by industrial scientists. It can be performed using either volumetric or coulometric measurement techniques.
 Image Credit: Mr.1 / Shutterstock
Image Credit: Mr.1 / Shutterstock
Principles of Karl Fischer titration
The KF reaction is based upon an early reaction called the Bunsen reaction, in which sulfur dioxide is oxidized by iodine with the consumption of water during this oxidation. The original reaction is as below:
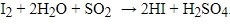
The KF titration reaction currently accepted is as follows, and was reached by several advances in understanding the mechanism of the reaction and modification of the original reagent:
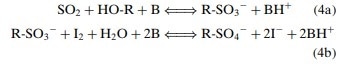
The titration reaction is said to reach its endpoint once the iodine stops reacting with the alkyl sulfite intermediate produced after the first half-reaction, resulting in excess iodine in the solution and a color change. This signals that no more water is present to be used up and therefore the reaction has stopped.
Water and iodine are consumed in a 1:1 molar ratio as shown by the equation. The end point of the titration is shown by a fall in the voltage required to pass a current through a double platinum electrode indicator. The voltage required for this current is high initially, but when iodine is in excess there is a sharp fall in this voltage.
The volume of reagent used up at this point is used to calculate the water content. With KF titration, a sample can be titrated from 100 to 1x106 ppm (water concentration from 0.01 – 100%). It has a high sensitivity and can thus measure even minute amounts of water. This holds good whether the water in the substance is free, emulsified or dissolved water.
Basics of volumetric and coulometric KF titration
KF titration occurs in two ways, either volumetric or coulometric. In short, the two methods may be compared as follows:
Volumetric titration
Reagent type
Volumetric titration uses the KF reagent containing sulfur dioxide and iodine, with an alkyl hydroxide and a base. This is first pretitrated so that any water contaminating the cell or dissolved from the air by the reagent is removed before the sample is added. The sample is then dissolved in the solvent. Through a two-step reaction the iodine reacts with sample water.
Reagent addition
The reagent is added drop by drop or even droplet by droplet using a buret, until the solution changes color, which is when there is excess iodine in the sample because all the water is used up already.
Samples with 0.1 to 500 mg of water can be measured this way using the right KF reagent. These reagents have specified titers which change over time and must thus be calibrated periodically using certified water standards. This method uses up more reagent but can handle larger sample volumes.
End point detection
This is detected by a bivoltammetric indicator electrode. The voltage required to keep polarizing current flowing at a defined value between the electrodes of the titration cell drops sharply once the end point is reached because of the presence of excess iodine. The titer of reagent added until this point is used to get the amount of water in the sample.
Coulometric titration
Reagent type
Coulometric titration is a highly accurate method of water determination. It is known as an absolute method. The coulometric cell contains two compartments, an anode and a cathode. It is extremely sensitive to the presence of water and therefore the cell must be impervious to any moisture from outside.
Pre-titration is therefore a lengthier process in coulometric KF titration. The sample is injected through a septum into the cell which is closed to the outside. The coulometric titration uses the coulometric KF reagent which contains iodide rather than iodine.
Reagent addition
With the coulometric method of KF titration, the titrant is produced within the titration cell itself by electrochemical reaction. The cell has a generator electrode polarized by alternating current to generate iodide instead of the titrating buret.
The iodide in the reagent undergoes oxidation to iodine at the anode, and the iodine then reacts with the sample water until the water is all used up. Once the end point is reached, the content of water is calculated from the amount of current required until this point. The benefits of using coulometric KF titration on samples with very little water include:
- better sensitivity
- higher speed of titration
- no need to calibrate the reagent each time as the iodine is produced in situ
- no need to replace the solvent each time
- multiple samples can be tested without reloading the reagent
- increased economy
End point detection
A very sensitive indicator is used, namely, a double platinum electrode indicator which uses alternating current. The amount of current passed to achieve sufficient electrolytic generation of iodine up to this point is measured and the corresponding water content is calculated.
Sample water range
Coulometric titration can detect even the small quantities of water present in gases. Usually a sample likely to have water content up to about 2% of sample, or 200 micrograms of water, is recommended, which would mean a total liquid sample size of 10 milliliters.
The ideal sample is one which contains less than 1% water, with 2 or less milliliters of sample in total, yielding less than 20 micrograms of water. Larger samples fill the cell too quickly and this makes cell cleaning and reagent reloading necessary, increasing the downtime. Volumetric titration is preferred for samples above 10 milliliters and 2% of water.
Solvent range
Coulometric determination is limited by the solvent range, and if appropriate solvents are not available a KF oven must be used to remove the water from the sample into another space where it is directly measured.
The need for the KF oven is therefore more limited with the volumetric method as a wider range of solvents can be used. In general, the volumetric method is preferred when the sample can be dissolved in the available solvents and is likely to contain less than 1% of water.
Further Reading